
The Center for Aerosol Impacts on the Chemistry of the Environment is an NSF-funded UCSD chemistry center headed by Dr. Kimberly Prather. After taking an Atmospheric Chemistry course in Fall 2019, I took an opportunity to volunteer in their lab, supervised by grad student Kathryn Mayer. I worked on Kathryn's project studying dimethyl-sulfide oxidation, using the PAM-OFR aerosol chamber. It was my first real lab experience (outside of classes) and my only involvement in atmospheric chemistry, before I shifted to physical oceanography work. Here's some info on what I learned:
Background
Background literature suggested that dimethyl-sulfide at the ocean surface oxidized mainly into methanesulfonic acid (MSA) and non-sea-salt sulfates, which were found to be present in resulting aerosol particles. Bates et al. (1992) suggested that the resulting ratio of MSA to nss-sulfates was 6.8%, and our main focus was to write a technical paper trying to replicate these results using the PAM-OFR aerosol chamber, to test whether or not it would be a viable instrument to simulate the marine headspace environment (the atmosphere just above the ocean surface).

Source: Andrew Lambe
The Potential Aerosol Mass - Oxidation Flow Reactor
The PAM-OFR, a little oversimplified, is a large (~40 liter) cylinder meant to serve as a simulated environment for aerosol reactions. There are inlets for various atmospheric gases, such as nitrogen and ozone, and for experimental gases and aerosols, in this case dimethyl-sulfide. After entering the chamber, interior UV lamps drive reactions. The conditions of the reactor can be tweaked to simulate any time span from 1 day to 30 days of aging in the atmosphere. The concept of "Potential Aerosol Mass" refers to the total possible aerosol mass that can be generated if the hypothesized reactions go to completion.
The resulting aerosols that flow out of the reactor are then collected on filters, which we dissolved in purified water to be analyzed by a mass spectrometer, courtesy of the UCSD ECAL lab.
One of my tasks in the lab was to run the PAM-OFR samples, which typically spent 24 hours collecting on the filter, after which I collected the filters and stored them so that they could later be analyzed.
Mass Spectrometry
Our method of finding out the ratio of MSA to sulfates was by mass spectrometry. Since we can't measure presence of molecules on the filter directly, we submerged the filters in purified water long enough that the mass on the filter could dissolved into an aqueous state. Small amounts of the resulting solution were then pipetted into MS vials to be sent through the spectrometer. The resulting output displays the relative abundance of different weights of materials in the solution, which can be compared with a library of known molecular signatures to identify how the ratios actually stacked up.

Source: ThermoFisher Scientific
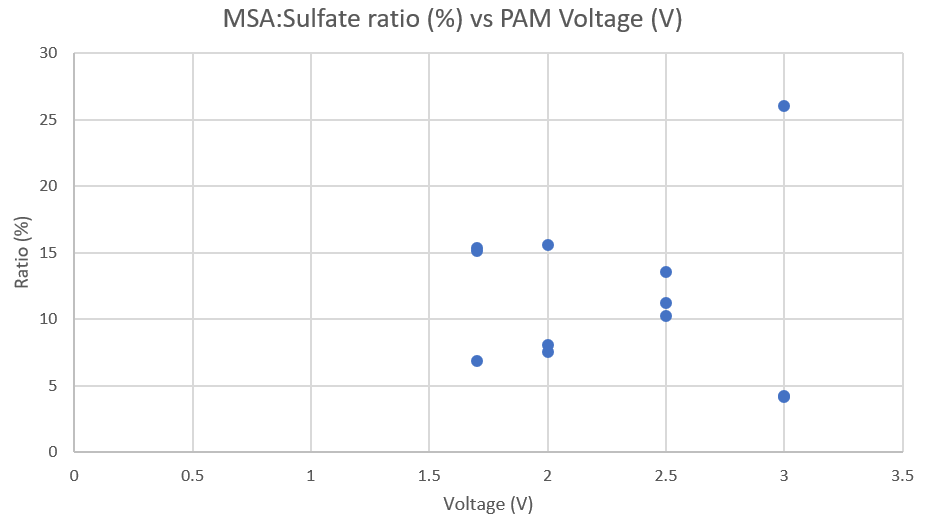
Results
The data from my time working on the project suggested some favorable results, showing ratios roughly around our expected values, while some were higher. My project got highlighted at Triton Day when our fluids professor J. Becker was presenting to prospective undergraduates about research done by students in the SIO department.
Conclusion
Unfortunately, we were close to finishing our lab work when the COVID-19 pandemic suddenly arrived and all UCSD labs shut down. Around this time I was beginning my spring quarter, and focusing more on my work with the Polar Center, so I decided it was best to search for other opportunities that would be more in line with my own field. Working with CAICE gave me some valuable experience with the nuances of planning and executing a research project, understanding and presenting my own knowledge, and lab skills that I want to translate to field work and observational oceanography.